Summary
Capsules are a widely used dosage form in both the nutraceutical and pharmaceutical industries, valued for their convenience, versatility, and consumer-friendly profile. This article provides an in-depth look at capsule types, benefits, applications, and considerations for businesses seeking OEM/ODM capsule production solutions.
What Are Capsules?
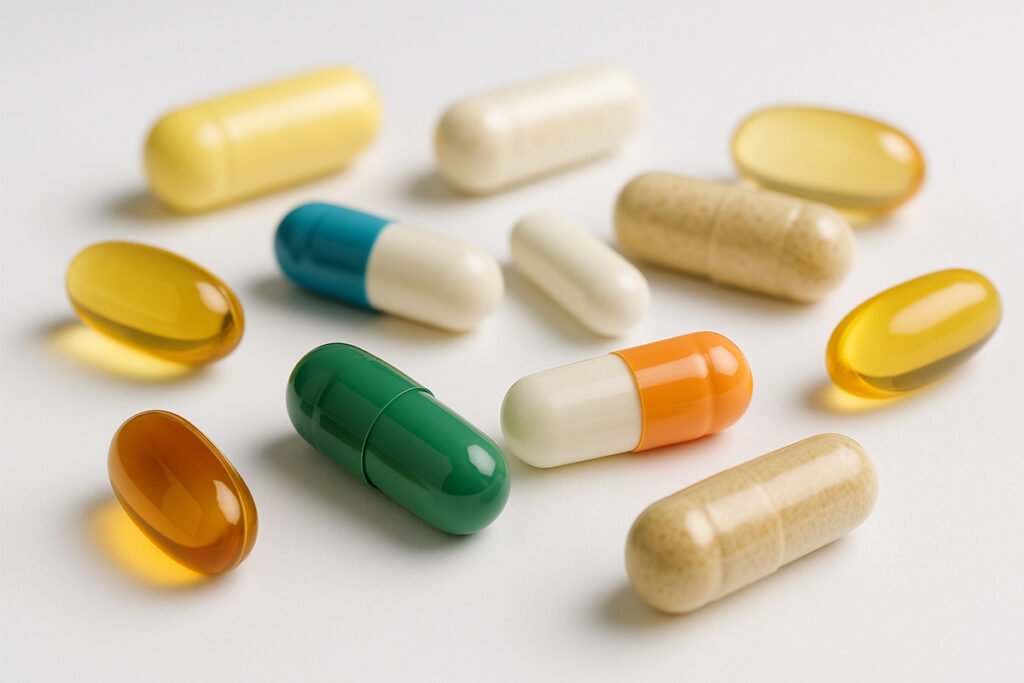
Capsules are solid oral dosage forms that enclose active ingredients within a gelatin or plant-based shell. Unlike tablets, which are compressed from powders, capsules are filled with various forms including powders, liquids, or granules. This structure offers better taste masking, easier swallowing, and increased bioavailability for many ingredients.
Capsules are typically available in two main forms: hard-shell capsules and softgel capsules. Each serves specific use cases depending on the nature of the ingredients, dosage requirements, and target consumer preferences.
Types of Capsules
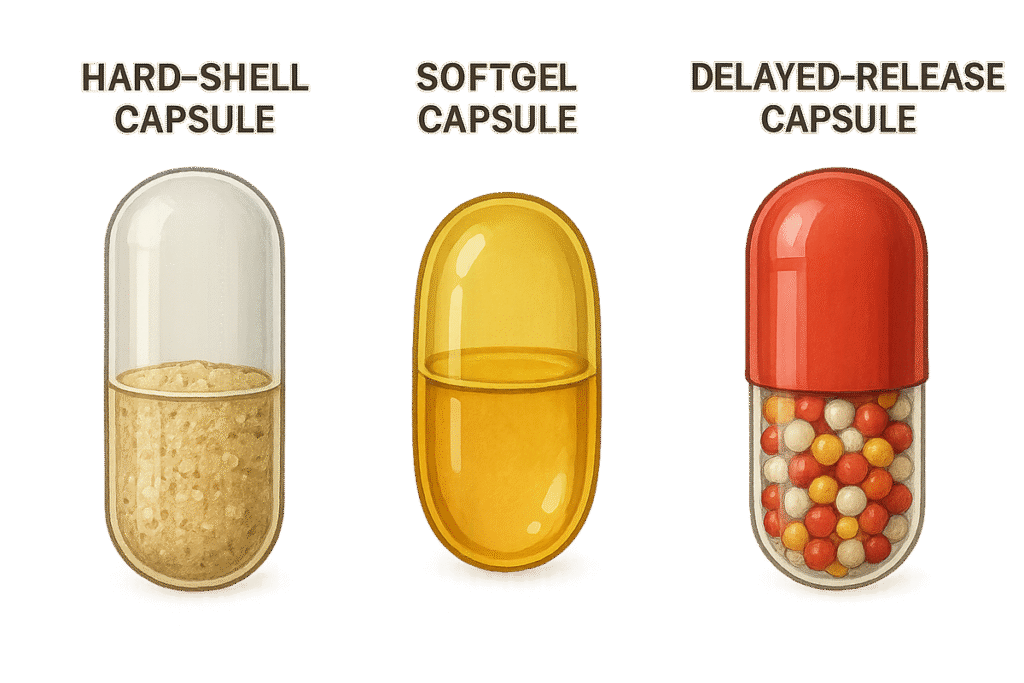
1. Hard-Shell Capsules
Also known as two-piece capsules, hard-shell types consist of a body and a cap. They are commonly filled with dry ingredients like powders, granules, or pellets.
- Materials: Gelatin or HPMC (vegetarian)
- Common Sizes: 000 (largest) to 5 (smallest)
- Applications: Herbal powders, vitamins, probiotics
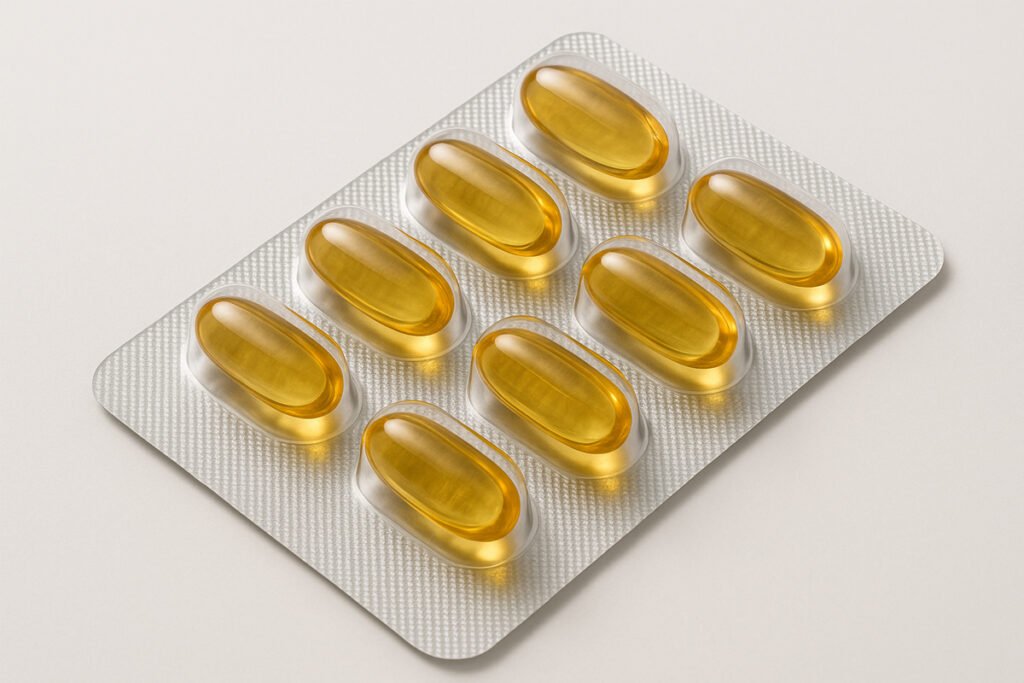
2. Softgel Capsules
Softgels are one-piece capsules usually filled with oils, emulsions, or lipid-based formulations. They provide high bioavailability and are ideal for fat-soluble compounds.
- Materials: Gelatin, carrageenan, or starch-based materials
- Applications: Omega-3, essential oils, fat-soluble vitamins
3. Delayed-Release Capsules
These capsules are specially coated to resist stomach acid and release contents in the intestine, ensuring targeted delivery.
Applications: Probiotics, enzymes, acid-sensitive compounds
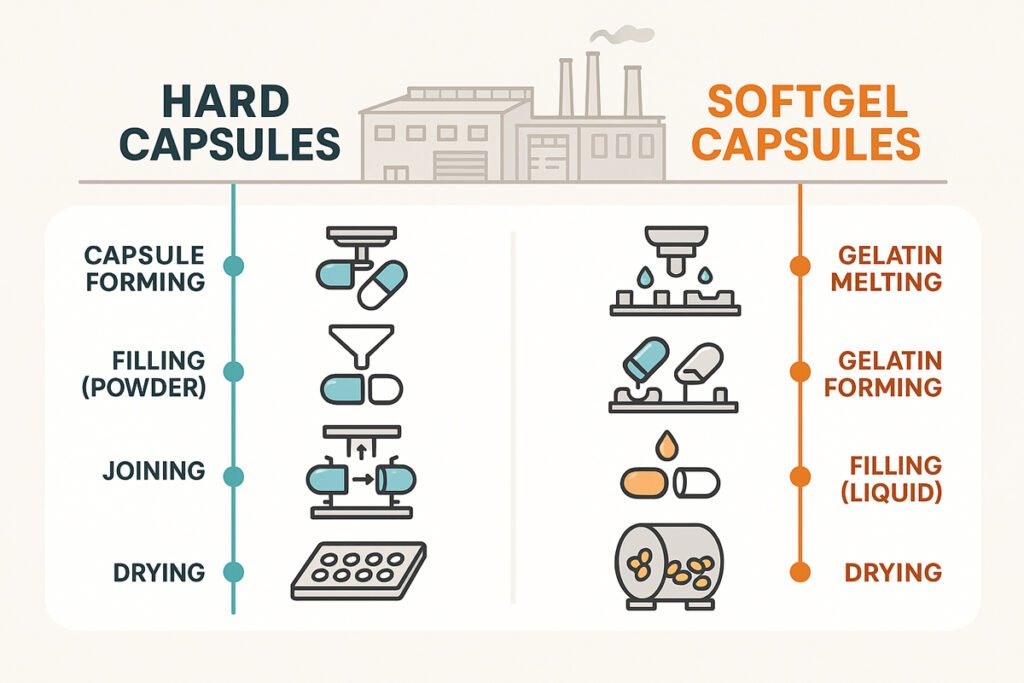
Capsule Manufacturing Process
Capsule manufacturing involves precise and tightly controlled steps to maintain product consistency and quality. For hard capsules, the process includes blending the fill material, automated filling, sealing, and inspection. Machines separate the cap and body, fill the powder or granules, and then rejoin the two parts.
For softgels, the process is more complex. It involves preparing a gelatin mass, forming capsule shells on a rotary die, simultaneous filling with liquid content, and sealing. This process requires exact temperature and humidity control to avoid shell deformation or leakage.
Both formats go through quality assurance testing, such as uniformity of weight, disintegration time, and microbial analysis.
Key Advantages of Capsules
Easy to Swallow: Especially compared to large tablets
Taste Masking: Enclosed shell prevents contact with unpleasant flavors
Flexible Formulation: Suitable for solids, liquids, or semi-solids
Quick Disintegration: Ensures faster absorption
Consumer Appeal: Clean appearance and smoother texture
Customization: Branding possible through color, size, or printing
Applications in Nutraceuticals
Capsules are extensively used in the health and wellness industry. They offer a practical way to deliver a wide range of dietary ingredients:
Functional Formulas: Sleep, energy, detox, immunity support
Herbal Extracts: Turmeric, echinacea, ginseng
Vitamins & Minerals: C, D3, B-complex, magnesium
Probiotics & Enzymes: Often protected by acid-resistant coatings
Applications in Pharmaceuticals
In pharmaceuticals, capsules offer reliable dosage accuracy and protection for sensitive actives. Common applications include:
Combination Therapies: Multi-drug dosing with separation inside the capsule
Pain Relief: Ibuprofen, acetaminophen
Antibiotics: Amoxicillin, doxycycline
Hormones: Progesterone, testosterone
Innovations and Market Trends
The demand for plant-based capsules is rising due to vegan, halal, and kosher market preferences. Materials like HPMC and pullulan offer vegetarian alternatives with excellent performance.
Emerging trends include:
- Liquid-in-capsule technology for enhanced nutrient absorption
- Time-release and enteric-coated designs for targeted delivery
- Two-in-one capsules (e.g., bead + powder) to deliver complex formulas
- Natural coloring agents for clean-label branding
Manufacturers are also focusing on sustainability and ingredient transparency, responding to global consumer demand for cleaner and more ethical supplements.

Packaging and Shelf Life
Capsules require packaging that protects them from moisture, light, and oxygen:
- Common formats: Blister packs, HDPE bottles, aluminum foil strips
- Storage conditions: 15–25°C with relative humidity of 35–55%
- Shelf life: Typically 18–36 months, depending on formulation and packaging
Incorporating desiccants and UV-resistant containers enhances product longevity.
OEM and ODM Considerations
If you’re exploring capsule production with a contract manufacturer, consider the following:
- Shell Type: Gelatin vs. vegetarian
- Filling Material: Powder, liquid, bead, or multi-phase
- Regulatory Standards: cGMP, FDA, ISO compliance
- Capsule Size & Color: Aligned with branding and dosage
- Label Design & Packaging: Private label and market-specific needs
- Batch Size & Lead Time: Especially important for new product launches
A reliable OEM/ODM partner should offer flexible MOQ, formulation assistance, and global compliance expertise.
Common Questions About Capsules
Q1: Are plant-based capsules effective as gelatin ones?
Yes. HPMC and pullulan capsules offer similar stability and performance, and they meet dietary and religious preferences.
Q2: Can capsules contain multiple ingredients?
Absolutely. Capsules can hold blends, pellets, or microencapsulated substances within the same shell.
Q3: What’s the difference between capsule and tablet shelf life?
Capsules often have a slightly shorter shelf life due to their moisture sensitivity, but proper packaging can equalize this difference.
Final Thoughts
Capsules offer an ideal balance of flexibility, functionality, and consumer acceptance. Whether you’re developing a dietary supplement or launching a pharmaceutical formula, capsules provide a professional and effective delivery format. Businesses interested in OEM or ODM services should evaluate capsule types, materials, manufacturing capabilities, and regulatory requirements to ensure a successful product launch.